Data or digital Revolution in the Pharmaceutical Industry ( Pharma 4.0 )
What is Industry evolution?
Industry evolution refers to the changes that occur in an industry over time, driven by factors such as technology, scale economies, and consumer demands. The evolution of an industry is determined by the selection mechanism, which is the process by which firms enter and exit, as well as the various factors that influence those decisions.
Industries have historically experienced multiple revolutions, starting with the First Industrial Revolution (Industry 1.0), followed by the Second, Third (digital revolution), and Fourth (smart machines, IoT). The Fifth Industrial Revolution (Industry 5.0) is the next phase of industrialization that builds upon Industry 4.0 and is enabled by developments in IT that include artificial intelligence, automation, big data analytics, the Internet of Things (IoT), machine learning, robotics, smart systems, and virtualization.
Industry 5.0 is focused on interaction among humans as well as machines, with robots and smart machines working alongside people with added resilience and sustainability goals included. The key pillars of Industry 5.0 are human-centric, resilient, and sustainable, with a focus on placing human creativity and well-being at the center of industry.
What is Industry 4.0?
The Fourth Industrial Revolution (4IR), sometimes referred to as “industry 4.0,” is the present period of digital revolution in production and manufacturing. The combination of cutting-edge technologies, including cloud computing, machine learning, artificial intelligence (AI), Internet of Things (IoT), and cyber-physical systems, is what defines it.
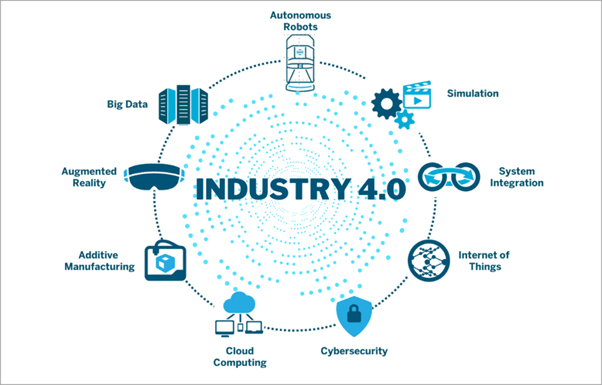
The above technologies facilitate the collection and analysis of real-time data in smart factories, hence resulting in enhanced automation, predictive maintenance, self-optimization, and higher efficiency in producing customized goods.
The convergence of operational technology (OT) and information technology (IT) systems is another key component of Industry 4.0, as it enables connectivity between production equipment and larger computer systems.
The adoption of smart manufacturing—which links digital technology, machine learning, and big data with physical production to create a more comprehensive and interconnected environment for businesses—is being propelled by this digital transition.
Industries making use of Industry 4.0
The industries using Industry 4.0 technologies include: Manufacturing, Pharmaceuticals, Transportation & Logistics, Chemical, Food & Beverage, Construction, Energy, Mining, Agriculture, Healthcare, Retail, Aviation, Textiles, Electronics, Metalworking, Automotive and Aerospace
To increase productivity, save waste, and improve product quality, these industries are embracing cutting-edge technologies including artificial intelligence (AI), cloud computing, advanced robotics, big data and analytics, additive manufacturing (3D printing), and the Industrial Internet of Things (IIoT).
What is Pharma 4.0?

In this extraordinary era, pharmaceutical manufacturers face numerous obstacles. There are stringent requirements for documentation, process validation, and data integrity to create an environment where compliance overshadows continuous improvement. But everything is not lost in this dire situation. Industry 4.0‘s ground-breaking new technologies, such robots, automation, and sophisticated analytics, have the power to completely change every facet of a pharmaceutical manufacturing lab. One can expect a significant change in this industry within the next five to ten years.
A full range of improvements could reduce quality control costs. In addition, accelerating issue solving through digitization and automation would lower manual error rates, improve quality, and guarantee adherence to industry standards.
Thanks to a new framework known as “Pharma 4.0,” pharmaceutical manufacturing may now operate in a more connected, flexible, and efficient manner. This is a novel approach to drug production that leverages data to swiftly address issues that arise during the manufacturing process.
Pharma 4.0 aims to bring about a culture revolution by promoting workflows that are more focused on the needs of people. Humans are at the center of pharmaceutical manufacturing thanks to this futuristic concept that connects workers through digital technologies.
The pharmaceutical industry embraces change by shifting tasks to smart laboratories and factories powered by information systems. Critical data-sharing processes aid in quicker decision-making and real-time systems optimization.
How is Pharma 4.0 different from previous industrial revolutions?
Pharma 4.0 differs from previous industrial revolutions in several ways:
Focus on Data and Analytics: Unlike earlier industrial revolutions, Pharma 4.0 prioritizes data collection and analysis to improve quality control, optimize production, and cut costs.
Integration of Advanced Technologies: Pharma 4.0 makes use of cutting-edge technologies like as AI, IoT, and Big Data Analytics, whereas previous industrial revolutions relied on simpler mechanical and electrical breakthroughs.
Regulatory Compliance: Pharma 4.0 considers the pharmaceutical industry’s specific regulatory compliance concerns, such as data integrity by design, which guarantees that data is acquired and kept in accordance with regulatory regulations. Previous industrial revolutions did not experience the same level of regulatory supervision.
Traceability and Transparency: Pharma 4.0 prioritizes traceability and transparency, which were not as important in past industrial revolutions.
Speed and Efficiency: Pharma 4.0 enables faster time to market and higher levels of efficiency when compared to prior industrial revolutions.
In conclusion, Pharma 4.0 distinguishes itself from previous industrial revolutions by relying heavily on modern digital technology, emphasizing data and analytics, and prioritizing regulatory compliance and traceability. Its purpose is to increase the entire efficiency and efficacy of the pharmaceutical sector, resulting in better patient care and safer drugs.
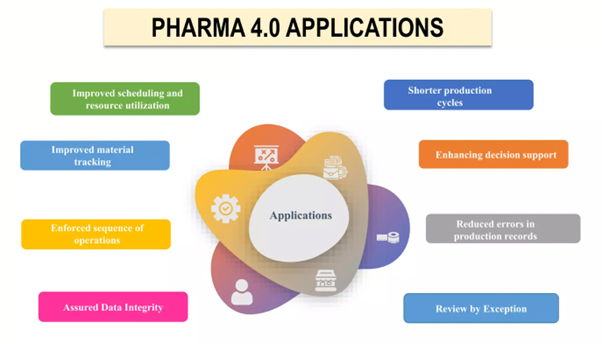
Real-world applications of Pharma 4.0 in the pharmaceutical and life sciences industries.
Below examples show how Pharma 4.0 is changing the pharmaceutical and life sciences industry by using sophisticated technology to create a more efficient, effective, and compliant manufacturing process and shows how Pharma 4.0 is transforming the pharmaceutical sector by employing cutting-edge technologies.
Digital analytics solutions: These solutions monitor processes, equipment, and raw material quality to detect anomalies that affect product quality before large volumes are created.
Predictive monitoring and maintenance: This technology detects the early indicators of part failure to save money on repairs, lengthen equipment life cycles, and avoid production losses.
Real-time visibility into operations: This provides insights into performance gaps, reduces bottlenecks, and improves agility for last-minute production modifications.
Distributed, automated testing: This moves product quality inspections upstream to detect and correct product quality and contamination before it reaches consumers.
Integrated supply chain: This provides complete transparency into the quality and quantity of raw materials and guarantees that suppliers satisfy specified requirements.
Smart glasses, augmented reality, and remote assistance tools: These enable safe, quick diagnosis and repairs.
Data-driven predictions: These let pharmaceutical businesses to simulate numerous situations and model their implications, resulting in better decision-making.
Autonomous production: Eliminates human error and improves employee safety by minimizing the need for employee to operate in dangerous conditions.
AI in drug discovery: AI-powered technologies speed the identification and validation of drug targets, resulting in faster and more accurate identification of possible novel therapies.
AI in drug development: AI predicts the safety and efficacy of new drug candidates, avoiding the need for costly and time-consuming clinical trials.
AI in manufacturing: AI optimizes production processes by predicting and preventing equipment breakdowns, reducing downtime.
IoT for supply chain visibility: IoT devices track items along the supply chain, offering real-time visibility and allowing for prompt responses in the event of quality issues.
Cloud computing for collaboration: Cloud-based platforms improve data exchange across organizations, allowing more efficient drug discovery and development.
Predictive maintenance: Predictive maintenance eliminates unexpected shutdowns and increases equipment longevity.
Remote communication technologies: Teleconferencing, videoconferencing, and virtual collaboration tools are examples of remote communication technologies that allow for real-time connectivity with stakeholders throughout the value chain.
Benefits of Pharma 4.0
Pharma 4.0 brings significant benefits to the pharmaceutical industry through digital transformation and advanced technologies. Listed below are few of the benefits:
Improved Quality: Pharma 4.0’s automation and constant monitoring result in improved production planning, lower errors, and higher-quality products and customer service.
Enhanced Compliance: Pharma 4.0 makes regulatory compliance easier by guaranteeing production process accuracy, lowering the possibility of creating substandard products, and facilitating regulatory adherence.
Faster Decision-Making: Pharma 4.0’s real-time monitoring and data analytics facilitate improved communication, expedited workflows, quicker decision-making, and on-time order delivery.
Cost Savings: Implementing Pharma 4.0 technologies requires a significant investment in infrastructure, software, and talent, but it can lead to cost savings through increased efficiency and reduced downtime.
Patient-Centric Approach: By utilizing cutting-edge technologies like AI, big data analytics, and IoT, Pharma 4.0 aims to make drug development faster, more efficient, and patient-centric.
Faster Time to Market: Advanced analytics and machine learning have the potential to reduce the time it takes for new medications to enter the market by accelerating the medication research and development process.
Data Privacy and Security: Ensuring data privacy and security is a major challenge, as the data is often sensitive and subject to regulatory scrutiny, but Pharma 4.0 technologies can help address these concerns.
Pharma 4.0 revolutionizes the pharmaceutical industry by driving digital transformation, improving productivity, ensuring quality, simplifying compliance, enabling faster decision-making, reducing costs, creating new business opportunities, and fostering a patient-centric approach to drug development.
Challenges of implementing Pharma 4.0
Like a coin with two sides, pharma 4.0 has challenges that need to be addressed for successful adoption. Listed below are few of the key challenges:
Technology Challenges: Incorporating new technologies and ensuring seamless integration across the organization poses a significant challenge. Data silos, legacy systems integration, and the need for advanced IT infrastructure to eliminate silos are key technological hurdles in Pharma 4.0 implementation.
Regulatory Compliance: Compliance with regulatory standards is a critical challenge in Pharma 4.0 due to the use of advanced systems and software. For pharmaceutical businesses, ensuring that new technologies adhere to regulatory requirements while protecting data privacy and security presents a substantial challenge.
Cost: Implementing Pharma 4.0 technologies requires a substantial investment in infrastructure, software, and talent. For smaller pharmaceutical companies, the expense of implementing cutting-edge technologies might be a major obstacle.
Protection of Intellectual Property: As digital technologies are used in pharmaceuticals more and more, it is important to safeguard intellectual property. Ensuring sufficient safety for physical components or AI systems’ algorithms presents issues that need to be continuously addressed.
Talent Acquisition and Retention: For Pharma 4.0, new skills like machine learning, artificial intelligence, and data analytics are essential. Employers may struggle to attract and retain employees with these specific skills, especially if there is a limited supply of highly qualified candidates.
How is pharma 4.0 changing the drug discovery & development process?
The integration of Pharma 4.0 technologies in drug discovery and development processes has profound impacts on various aspects of the pharmaceutical industry, leading to significant improvements in efficiency, accuracy, and innovation. Here are some key impacts:
- Accelerated drug discovery through advanced analytics, AI, and machine learning.
- Improved target identification and validation, leading to more successful drug candidates.
- Enhanced preclinical and clinical trial design, resulting in shorter development timelines and reduced costs.
- Facilitation of personalized medicine development, tailored to individual patient characteristics.
- Opportunities for drug repurposing through data-driven approaches.
How is pharma 4.0 impacting clinical trials?
- Accelerated patient recruitment and retention through advanced analytics and remote monitoring technologies.
- Optimization of clinical trial protocols using predictive modeling and adaptive trial designs.
- Real-time data monitoring and analysis, enabling faster decision-making and adaptive strategies.
- Enhanced safety monitoring through automated adverse event detection and signal management.
How is pharma 4.0 impacting regulatory compliance and safety?
- Enhanced regulatory compliance through data analytics and real-time monitoring of manufacturing processes.
- Improved drug safety monitoring, with proactive identification of adverse events and potential risks.
- Streamlined regulatory submissions and approvals, leveraging digital platforms and standardized data formats.
How is pharma 4.0 impacting manufacturing and supply chain?
- Optimization of manufacturing processes through automation and robotics, leading to increased efficiency and reduced errors.
- Improved supply chain management, with enhanced transparency and traceability using IoT sensors and blockchain technology.
- Real-time monitoring of production and distribution, ensuring compliance with regulatory standards and minimizing waste.
How is pharma 4.0 impacting research and innovation?
- Accelerated innovation through collaboration, data sharing, and interdisciplinary approaches.
- Increased efficiency in research and development processes, with streamlined workflows and optimized resource allocation.
- Facilitation of data-driven research initiatives, driving discoveries and breakthroughs in life sciences.
- Expansion of scientific knowledge and understanding through advanced analytics and computational modelling.
The adoption of Pharma 4.0 in the pharmaceutical and life sciences industry leads to transformative impacts across various domains, including drug discovery, manufacturing, clinical trials, regulatory compliance, patient engagement, and research innovation. These impacts contribute to improved healthcare outcomes, increased efficiency, and enhanced sustainability in the industry.
The pharmaceutical industry is developing rapidly. The promise of Pharma 4.0 lies in its ability to unlock new opportunities for productivity and quality, and to make compliance a seamless part of the manufacturing process. Manufacturers who take the necessary steps to embrace the digital future now benefit from seamless compliance, but it doesn’t have to stop there. By implementing a Pharma 4.0 strategy, you can increase relationships, efficiency, and flexibility.
About the Author :
Business Development Executive – Pharma & Life Sciences
Ms.Deepthi Chatterjee is Bestowed with the following Licenses &
Certifications :
https://www.linkedin.com/in/de












