GenAI – Driven Validation in Life Sciences : Ensuring GxP Compliance through Data Governance and Automation
Executive Summary
In the highly regulated life sciences industry, ensuring compliance with Good x Practice (GxP) standards is essential for maintaining product safety, efficacy, and quality. Generative AI (GenAI) offers a transformative solution to the traditional Computer Software Validation (CSV) and Computer Systems Assurance (CSA) processes. This white paper explores how GenAI can automate and streamline validation workflows, improving the speed, accuracy, and compliance of critical GxP processes.
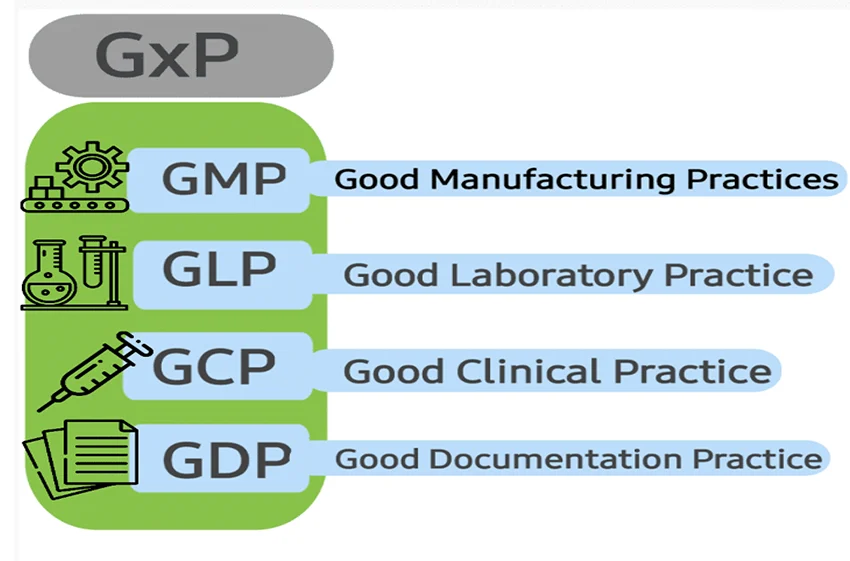
By leveraging Generative AI for GxP compliant validation, data governance, and enterprise workflows, life sciences organizations can automate complex validation tasks, ensure real-time compliance, and achieve regulatory readiness with significantly reduced manual effort and human error. The integration of AI into Watsonx, cloud systems, and enterprise software further enhances the scalability, flexibility, and transparency required to meet evolving GxP standards.
 Introduction
Introduction
Compliance with GxP regulations is essential in the life sciences sector to ensure the safety and effectiveness of pharmaceutical and medical products. Compliance verification and software validation have traditionally been tedious, resource-intensive tasks. Companies often rely on manual processes, static documentation, and periodic reviews, leading to inefficiencies, increased costs, and a higher risk of non-compliance.
Traditional CSV and CSA processes involve rigorous testing, documentation, risk assessments, and system updates that are slow and prone to human error. Additionally, regulatory environments are constantly evolving, creating a need for dynamic validation practices that are agile and efficient.
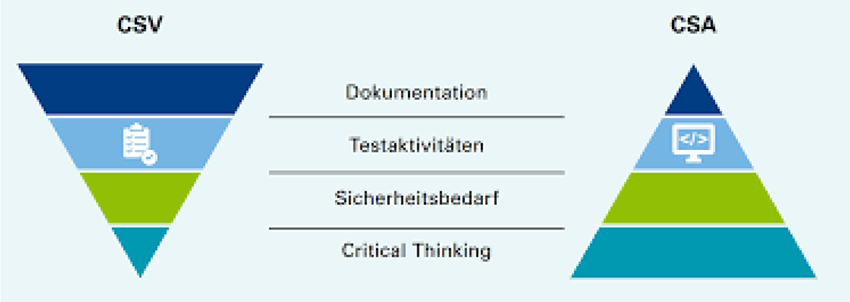
Generative AI can drive these processes with automation, real-time validation, intelligent risk management, and continuous compliance monitoring. This white paper will discuss the role of GenAI in ensuring GxP compliance through improved data governance, enterprise workflow integration, and automated validation practices.
Problem Statement

The life sciences industry faces several challenges when it comes to ensuring GxP compliance and validation:
➢ Manual Validation Processes: The traditional approach to CSV and CSA is largely manual, relying on repetitive checks, human intervention, and static documentation. This increases the risk of errors and delays, impacting operational efficiency and time-to-market.
➢ Complexity in Regulatory Compliance: GxP compliance requirements vary across regions and evolve over time. Keeping up with these changes and ensuring that the systems and processes are continuously compliant is a constant challenge for life sciences organizations.
➢ Data Integrity Issues: Data generated through different systems (e.g., ERP, LIMS, MES) needs to be accurate, traceable, and secured. Ensuring data integrity throughout the validation process remains an ongoing challenge.
➢ Scalability: As organizations scale, traditional validation processes become increasingly difficult to manage across multiple systems, applications, and geographies.
Business Case for AI-Driven Validation
The adoption of GenAI-driven validation offers several compelling business advantages:
➢ Improved Efficiency and Cost Savings: Automating manual tasks like validation documentation, testing, and risk assessments reduces the need for extensive human labor, resulting in significant cost savings and faster validation cycles.
➢ Enhanced Compliance: GenAI systems continuously monitor regulatory updates and ensure that software and systems remain compliant in real-time, reducing the risk of non-compliance.
➢ Faster Time-to-Market: The automation of validation workflows speeds up regulatory approvals, allowing for faster product development and quicker entry into the market.
➢ Reduced Human Error: AI-based validation reduces the risk of human error by ensuring consistent and accurate documentation, testing, and reporting throughout the validation lifecycle.
➢ Scalability and Flexibility: As companies expand their operations or adapt to new regulations, GenAI can scale across systems, applications, and geographies, ensuring compliance at every stage.
How Generative AI contributes to GxP Validation
Generative AI can transform CSV and CSA processes by automating key validation steps, reducing manual effort, and ensuring compliance. Below are some ways GenAI helps enable GxP-compliant validation:
➢ Automated Document Generation
One of the most labor-intensive tasks in GxP validation is the creation of documentation to prove compliance with regulatory requirements. These include:
• Validation plans
• Test protocols
• Test reports
• Risk assessments
• User Requirement Specifications (URS)
• System Specifications (SRS)
Generative AI can help automate the creation of these documents by understanding the context of the system and the specific regulatory requirements. For example, it can generate test protocols based on the system’s design and regulatory guidelines, ensuring that all necessary tests are covered.
Example: A pharmaceutical company uses Generative AI to automatically generate a validation plan for a new laboratory automation system. The AI draws from historical data on similar systems and guidelines to create a tailored document that adheres to GxP.
➢ Automated Test Script Generation
Generative AI can automatically generate test scripts for functional, performance, and stress testing of software and systems. These test scripts are key to the verification and validation process, ensuring that software or systems are operating according to specifications and can meet the required operational standards.
Example: In the context of a manufacturing execution system (MES), Generative AI could analyse the user requirements and automatically generate test scripts that validate data entry, data storage, and report generation, ensuring that the system meets GxP requirements for data integrity.
➢ Simulation and Risk-Based Testing
Generative AI can simulate different scenarios to validate software or system behaviour, ensuring that it will work correctly in real-world conditions. Risk-based testing, a common practice in GxP validation, can be further enhanced by AI by automatically identifying areas of risk and ensuring that critical functionalities are thoroughly tested.
Example: AI could simulate a failure in the production environment and predict how the system would behave. It could then automatically generate test cases to ensure that the system is robust against such failures, which would be critical for GxP compliance.
➢ Data Integrity Monitoring and Validation
Generative AI can automate data integrity checks by monitoring data as it is created, transferred, and stored across systems. By continuously evaluating data against predefined rules, AI can ensure that no data discrepancies occur, ensuring the traceability and accuracy required by GxP guidelines.
Example: In a clinical trial data management system, AI can monitor incoming data from various sources (e.g., test results, patient reports) and automatically flag any inconsistencies, missing data, or potential errors, ensuring compliance with GxP standards for clinical data integrity.
➢ Change Control Automation
GxP-compliant systems must have strict controls on changes to software, systems, or processes. Generative AI can assist in ensuring that any changes are validated according to GxP standards by automating change control documentation, impact analysis, and validation of the changes.
Example: When a software update is deployed to a manufacturing system, AI can automatically validate whether the update complies with the GxP requirements by checking the software against the original system specification and generating relevant validation reports.
➢ Continuous Monitoring and Reporting
Generative AI can be used to automate continuous system monitoring and generate real-time reports on system performance and compliance. This proactive approach helps in early detection of issues that may affect GxP compliance and can reduce the cost and time spent on manual audits.
Example: A manufacturing system can be monitored by AI for any anomalies in production data (e.g., temperature, humidity, or equipment malfunction), and the AI would automatically alert the quality control team, generate a non-compliance report, and suggest corrective actions.
➢ Continuous Compliance Monitoring
Ongoing Validation: AI continuously monitors the system’s performance against compliance requirements, identifying deviations or non-compliance issues in real-time and triggering alerts or corrective actions.
Example: In a clinical trial management system, GenAI continuously checks compliance with regulatory standards, sending alerts to QA teams if a test case fails or a critical parameter is missed.
Data Governance with Watsonx
IBM Watsonx is a platform that helps manage data governance, integrity, and security across AI-driven validation processes. Key features include:
➢ Centralized Data Repository: All compliance-related data, including system configurations, test cases, and validation reports, are stored in a single, secure, centralized repository.
➢ Data Integrity Checks: Watsonx ensures that all data used in validation processes is accurate, secure, and traceable. It automatically detects discrepancies and triggers corrective actions.
➢ Automated Audit Trails: Watsonx generates an immutable audit trail for every action taken during the validation process, making it easier to maintain compliance records for regulatory reviews.
Example: In a clinical manufacturing environment, Watsonx tracks every data point from batch testing, ensuring complete traceability from raw materials to finished products.
Integration into Enterprise Workflows
Integrating GenAI-driven validation into existing enterprise workflows is critical for ensuring seamless and efficient validation processes. Here’s how GenAI integrates with key systems:
Example 1: Integration with ERP Systems (SAP)
AI for Automated Purchase Orders: When a new product is ready for production, GenAI can analyse inventory levels and create purchase orders for raw materials automatically, ensuring that the production process is compliant with regulatory standards.
Example 2: Integration with LIMS and MES
AI for Test Case Execution: AI integrates with Laboratory Information Management Systems (LIMS) and Manufacturing Execution Systems (MES) to automatically run test cases based on predefined validation protocols.
Results and Impact
By adopting GenAI-driven validation, life sciences organizations experience several tangible benefits:
➢ Reduced Validation Time: Automation accelerates test case generation, execution, and documentation, reducing validation cycles by up to 50%.
➢ Improved Compliance: Continuous monitoring and real-time validation ensure compliance with GxP standards, resulting in fewer audit findings and regulatory penalties.
➢ Cost Savings: The reduction in manual efforts and rework lowers the overall cost of validation, with up to 40% savings in labor and compliance costs.
➢ Faster Time-to-Market: By streamlining the validation process, companies can bring products to market faster, ensuring a competitive advantage.
Conclusion
Generative AI provides a transformative solution for CSV and CSA processes in life sciences. By automating validation workflows, ensuring continuous compliance, and improving data governance, GenAI enables life sciences companies to streamline validation tasks while ensuring they meet regulatory requirements. Integrating GenAI with enterprise systems like IBM Watsonx, LIMS, and MES enhances real-time compliance monitoring, risk assessments, and automated documentation generation, resulting in faster time-to-market, reduced costs, and improved audit readiness.
About the author:

Ms. Deepthi Chatterjee
Business Development Executive (Pharma & Lifesciences Industry)
IBM India Pvt. Ltd

Ms. Deepthi Chatterjee is Bestowed with the following Licenses & Certifications:
https://www.linkedin.com/in/de
Ms. Deepthi Chatterjee can be contacted at :











